The Telangana Drugs Control Administration (DCA) has issued a Stop Use Notice for two cough syrups after laboratory analysis confirmed the presence of Diethylene Glycol (DEG) — a highly toxic chemical compound known to cause severe health complications and even death when consumed.
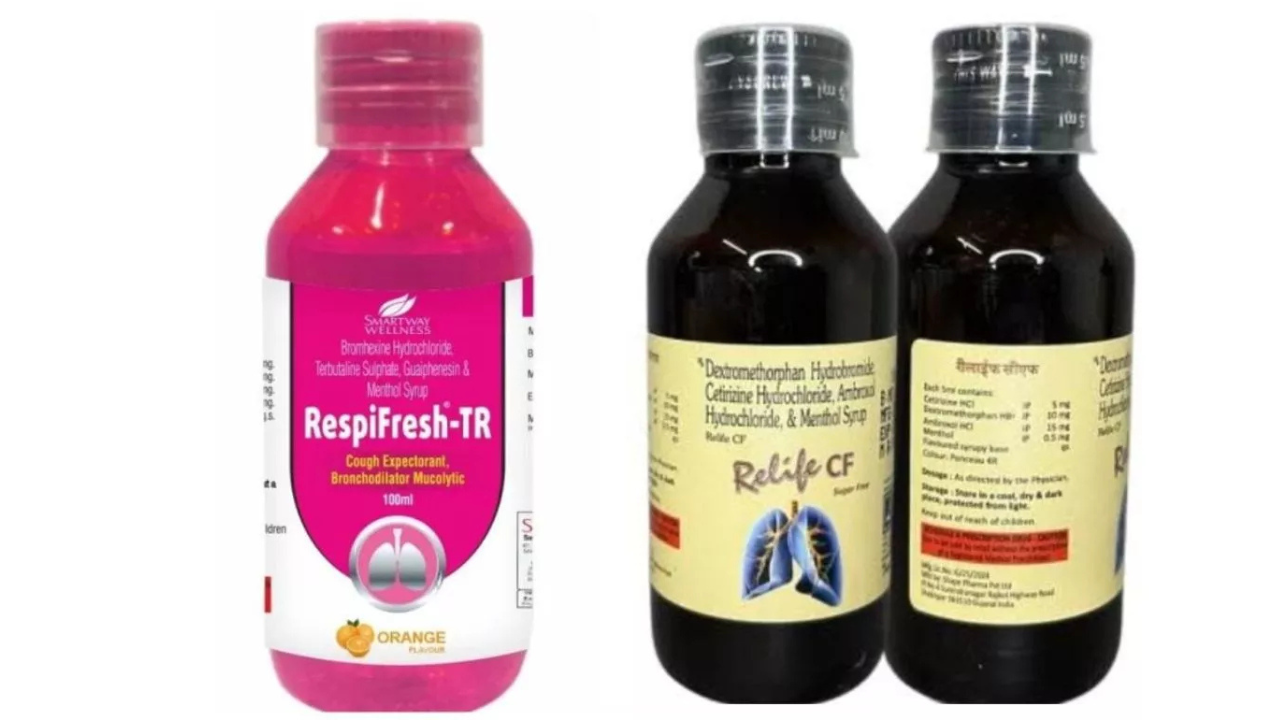
The alert was issued following a report from the Drug Testing Laboratory in Madhya Pradesh, which declared that Relife Cough Syrup and Respifresh TR Cough Syrup were contaminated with DEG.
The affected products are:
- Relife Cough Syrup containing Ambroxol Hydrochloride, Guaiphenesin, Terbutaline Sulphate, and Menthol
- Batch Number: LSL25160
- Manufacturer: Shape Pharma Private Limited, Gujarat
- Expiry: December 2026
- Respifresh TR Cough Syrup containing Bromhexine Hydrochloride, Terbutaline Sulphate, Guaiphenesin, and Menthol
- Batch Number: R01GL2523
- Manufacturer: Rednex Pharmaceuticals Private Limited, Gujarat
- Expiry: December 2026
Public Alert Issued
The DCA has urged the public to immediately stop using these two cough syrups and to report any possession of them to the nearest Drugs Control Authority. Citizens can also contact the DCA through its toll-free helpline 1800-599-6969, available between 10:30 a.m. and 5:00 p.m. on working days.
Following the alert, all Drugs Inspectors and Assistant Directors across Telangana have been instructed to alert pharmacies, distributors, wholesalers, and hospitals to freeze existing stocks of the mentioned batches. The DCA has ordered that these medicines must not be sold, dispensed, or distributed under any circumstances.
About the Chemical and Health Risks
Diethylene Glycol (DEG) is an industrial solvent used in products like antifreeze and brake fluids. It is not intended for medicinal use. When consumed, DEG can cause acute kidney failure, metabolic acidosis, neurological damage, and even death.
Health experts warn that exposure to DEG-contaminated medicines can lead to symptoms such as vomiting, abdominal pain, reduced urine output, drowsiness, or seizures. Immediate medical attention is advised if such symptoms appear after taking cough syrup.
Previous Incidents and Wider Action
This warning follows similar alerts issued earlier this year after another cough syrup, Coldrif Syrup, was linked to multiple child deaths in Madhya Pradesh and Rajasthan. Laboratory tests had shown that the Coldrif batch was also contaminated with Diethylene Glycol.
The repeated detections of DEG across different cough syrup brands have raised serious concerns about drug manufacturing safety and quality control in India. The Central and State Drug Control Authorities are now conducting nationwide inspections, sample testing, and audits of pharmaceutical companies to identify and prevent further contamination incidents.
Public health experts have called for stricter enforcement of Good Manufacturing Practices (GMP) and stronger oversight of raw material sourcing to ensure the safety of over-the-counter medications, especially those widely used by children.
What Consumers Should Do
- Check cough syrup bottles for the following batches:
- Relife Cough Syrup – Batch No. LSL25160
- Respifresh TR Cough Syrup – Batch No. R01GL2523
- Stop usage immediately and hand over the product to the nearest drug control office.
- Report details by calling 1800-599-6969 during office hours.
- Seek immediate medical attention if anyone who consumed these syrups shows signs of illness.
Conclusion
The Telangana DCA’s proactive action aims to prevent further health risks arising from contaminated medicines. Citizens are advised to remain alert, verify medicine batch numbers before consumption, and report any suspicious or unverified medical products to authorities.
The incident has once again highlighted the urgent need for stricter pharmaceutical regulation, transparent quality testing, and swift public communication to ensure the safety of healthcare consumers across India.
